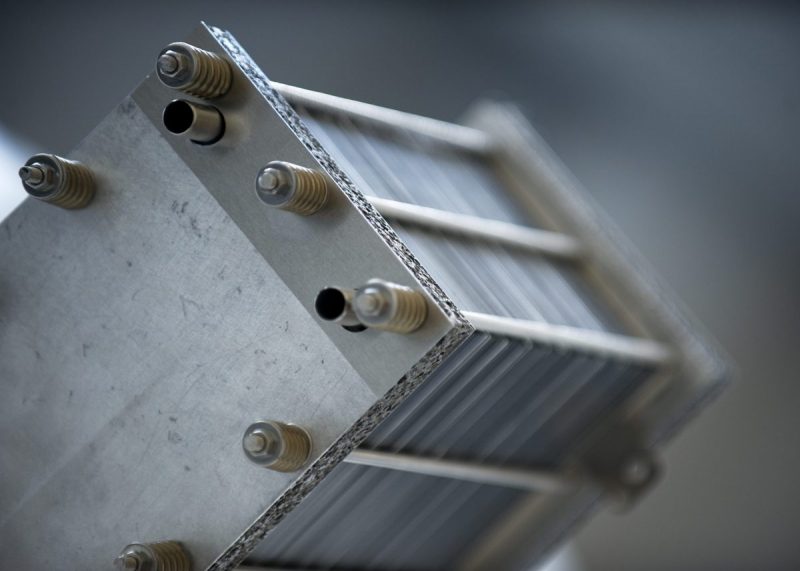
Fuel cells in cars have been discussed for years. For power supply, they are often used as an alternative to diesel generators. But what is a fuel cell? How does it work? And which applications rely on them?
1. What are fuel cells and what are they used for?
2. Simply explained: How fuel cells work
3. Fuel cell advantages and disadvantages
- Off-grid, backup power, residential energy: Fuel cells in stationary applications
- Cars and vehicles: fuel cells in mobility applications
What are fuel cells and what are they used for?
A fuel cell is a device that generates electrical and thermal energy through the use of a fuel. From the outside, fuel cells hardly differ from combustion engines. Unlike an internal combustion engine, the fuel in the fuel cell is not burned, but converted into electricity and heat by means of a chemical reaction.
Nowadays, fuel cells are used primarily for power supply. One of the advantages is their zero-emission operation – thus serving as an environmentally friendly alternative to internal combustion engines. Another advantage is that the waste heat generated can easily be used for heating.
Fuel cells can also be used effectively in mobility. On the one hand, they provide high energy density while, on the other, permitting rapid refilling of the fuel tank. In the commercial vehicle sector, fuel cells are an essential component of future mobility – not as an alternative, but in combination with batteries.
In applications where large amounts of energy are required and space comes at a premium, batteries alone face technical limitations. The weight is high, as is the space requirement. For commercial applications and heavy-duty vehicles, this calls into question the everyday suitability of direct electrification via batteries
Simply explained: How fuel cells work
Fuel cells convert the chemical energy of a fuel directly into electrical energy (and heat). This process is called an electrochemical reaction. In contrast to batteries, fuel cells are not energy storage devices, but energy converters.
![]() More on the topic of energy storage
More on the topic of energy storage
Energy can occur in different physical forms. Chemical energy is stored in the form of chemical compounds in an energy carrier (e.g. fuel) and can be released during reactions. This effect is used, for example, in the combustion of fuels in vehicles. When internal combustion vehicles are driven, chemical energy is converted into mechanical energy and heat by burning the fuel. The energy density of the fuel is a decisive factor. An overview can be found below in the text.
Design and principle
Each fuel cell consists of 2 electrodes (anode and cathode) separated by an electrolyte. The electrodes are conductive for electrons, while the electrolyte is only permeable for a certain type of ions (electrically charged atoms). The diagram shows the structure and function of a hydrogen-oxygen fuel cell:
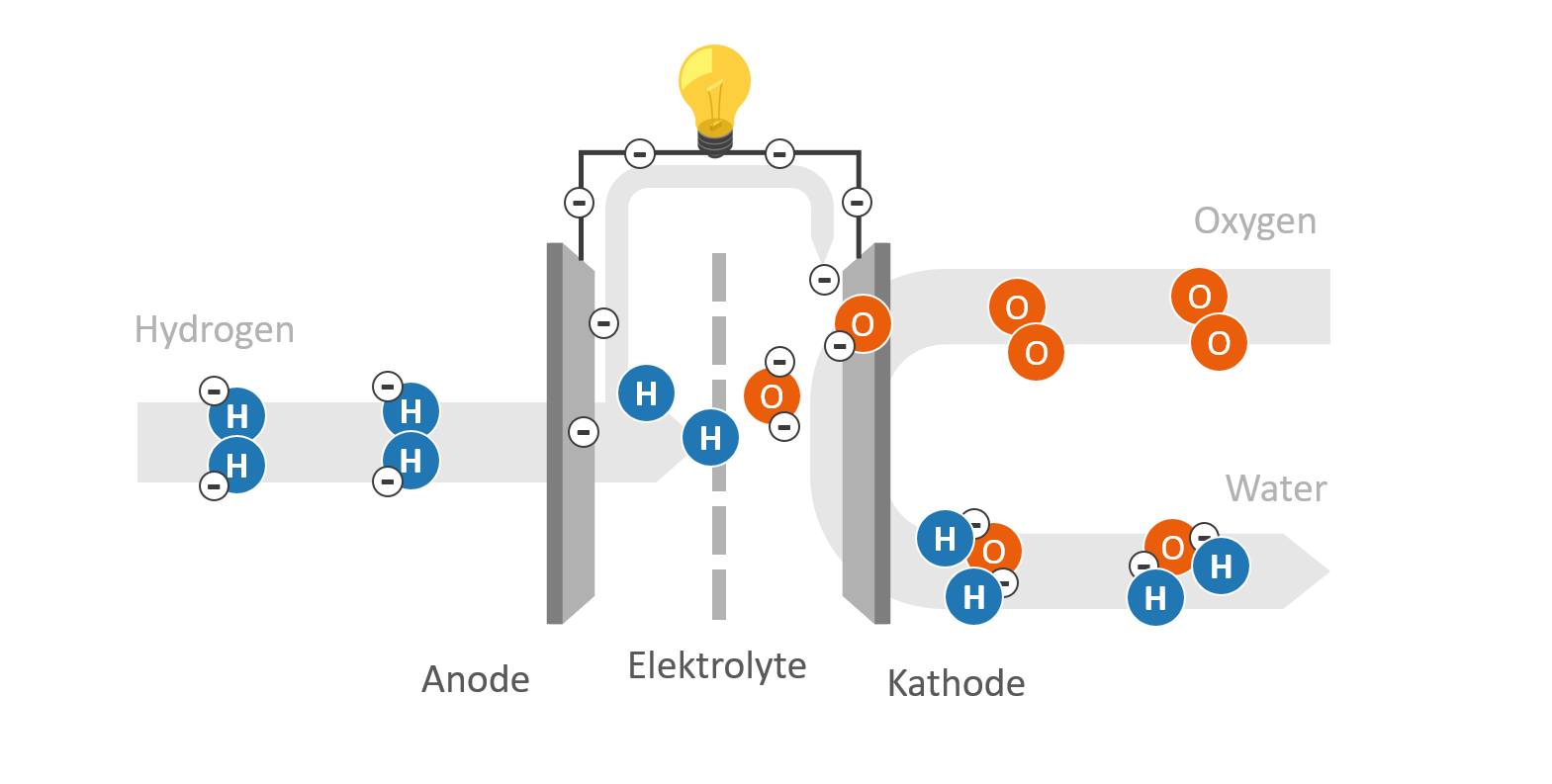

Principle and basic design of a fuel cell – Schematic illustration
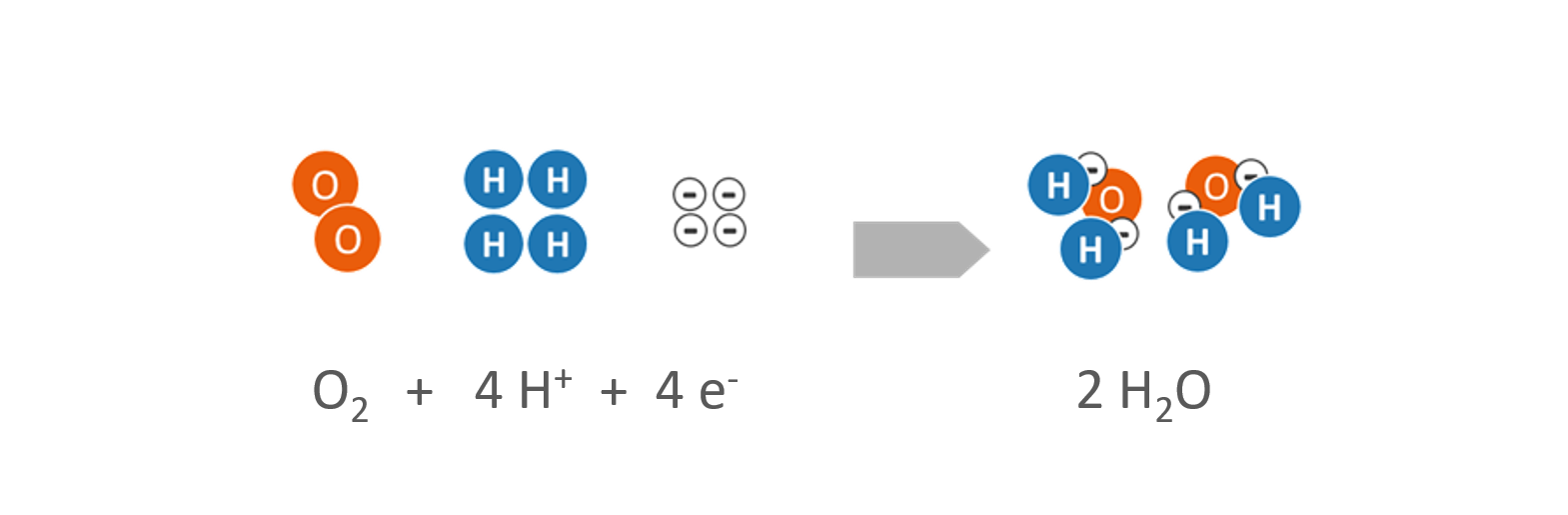
- Hydrogen (H2) is supplied to the anode as a fuel. By help of a catalyst, the hydrogen is separated into positively charged hydrogen protons (H+) and negatively charged electrons (e–).
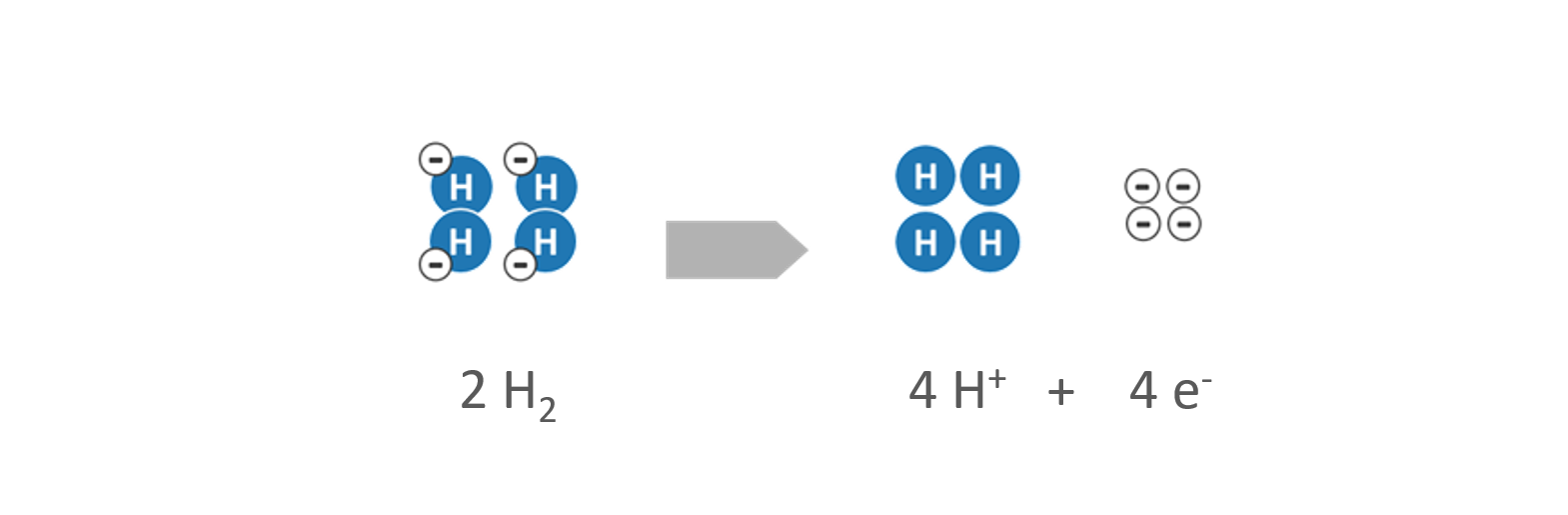
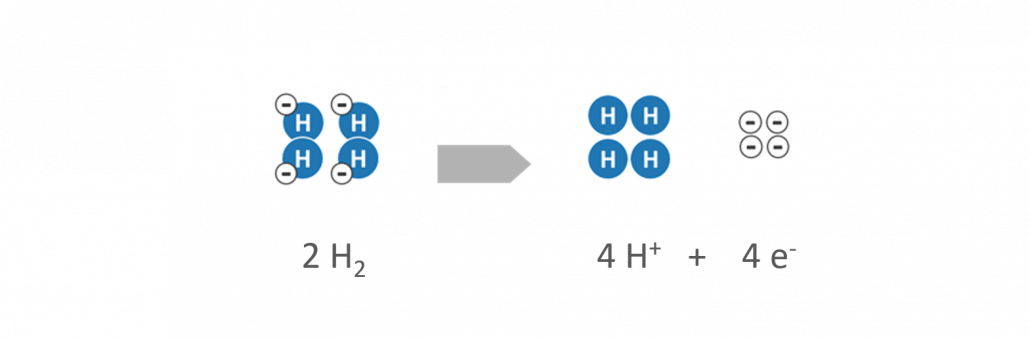
Reaction at the anode
- Oxygen (O2) is supplied at the cathode. The oxygen is usually derived from the ambient air.
Reaction at the cathode
- Both hydrogen protons (H+) and electrons (e–) migrate to the cathode, where they react with the added oxygen (O2) to form water (H2O).
The electrolyte prevents the released electrons (e–) from reaching the cathode directly. The electrons (e–) are thus forced to flow through an external conductor. If a load (e.g. a light bulb) is integrated into the electrical circuit, the electrons emit electrical energy.
Dependent on the operating point, a single cell supplies a specific voltage. This is usually between 0.5 – 1.0 volts. For higher voltages, individual cells are connected in series. A connection of several cells is called stack, which builds the core element each fuel cell system.
Power, efficiency, and lifetime
Factors such as power, efficiency and lifetime depend primarily on the fuel used and the type of fuel cell employed. In general, there is a correlation between the size of the fuel cell stack and the power output.
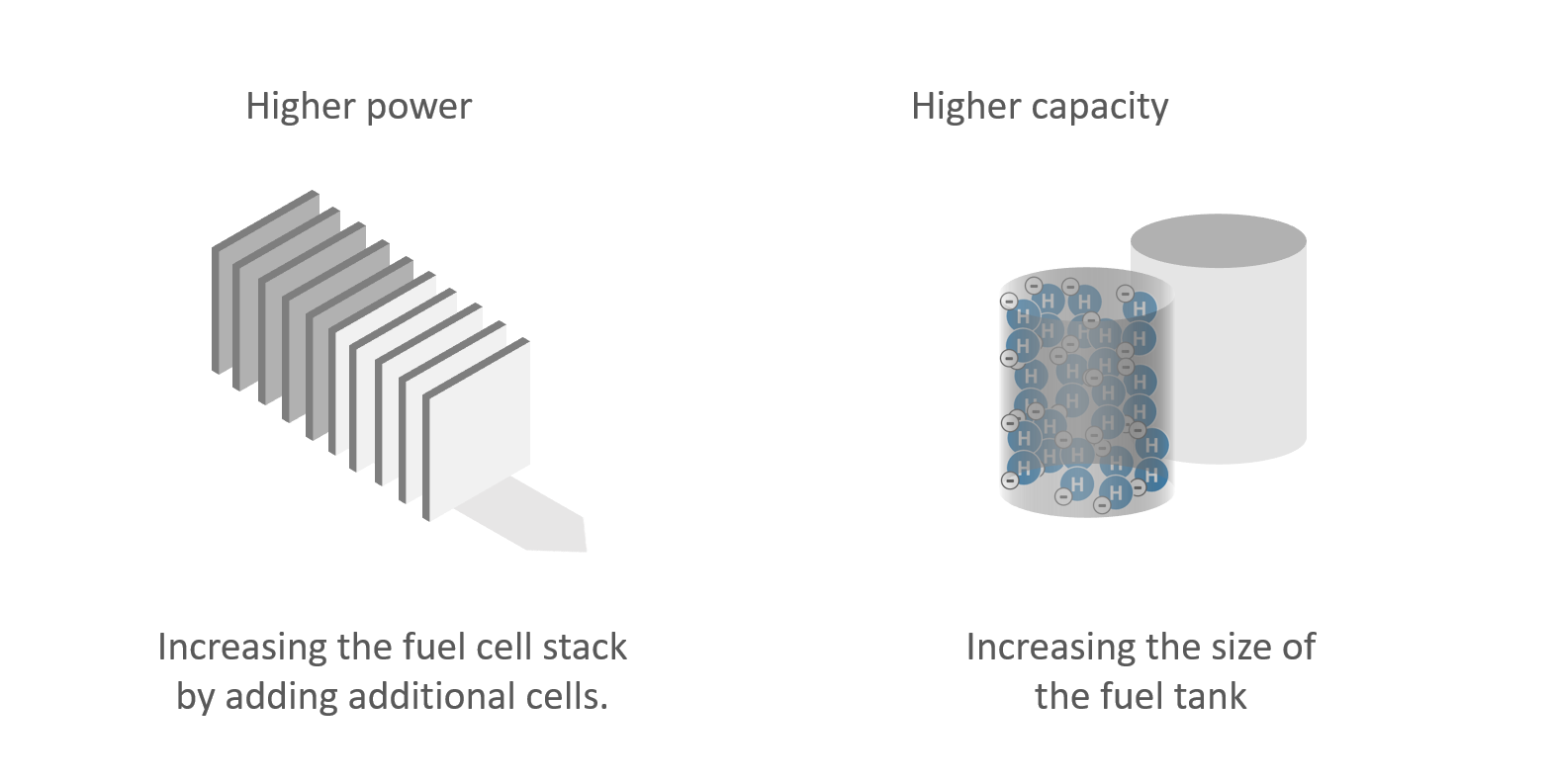

Fuel cell: Relationship between capacity and power
The capacity of a fuel cell can be increased via the external tank. The tank is thus comparable to a battery, which limits the total amount of energy that can be generated via the fuel cell if not refilled. Capacity and actual power output of a fuel cell are independent of one another.
![]()
![]()
![]()
When designing energy systems, two parameters are crucial: capacity and power. Although their units “kilowatt-hour (kWh)” and “kilowatt (kW)” sound similar, the terms are to be used differently:
Capacity describes the size of an energy storage device and consequently the total amount of electrical energy within the system. A battery with a capacity of 4 kWh can provide 4 kWh of electrical energy before it has to be recharged. The capacity is thus directly linked to the electrical consumption.
Power describes the amount of energy required at a single moment. A hair dryer requires an output of 2,000 watts (2 kW) to function. If the hair dryer runs continuously for one hour, 2 kWh of electrical energy is required (2 kW x 1 h). If the hair dryer runs for 3 minutes, 0.1 kWh is required (2 kW x 0.05 h).
Although most fuel cells run on a reaction of hydrogen and oxygen, there are differences in terms of design. Generally, fuel cells are classified based on the electrolyte that is used. The electrolyte determines the operating temperature and other properties of the cell.
The higher the operating temperature, the greater the tolerance to impurities and contaminants within the fuel (e.g. carbon dioxide or carbon monoxide). On the other hand, with higher temperatures the demands on the system technology and materials used increases as well, as does the time required for startup.
An overview of the main operating parameters and types of fuel cells can be found in the following section.
Fuel cell technology and types of fuel cells
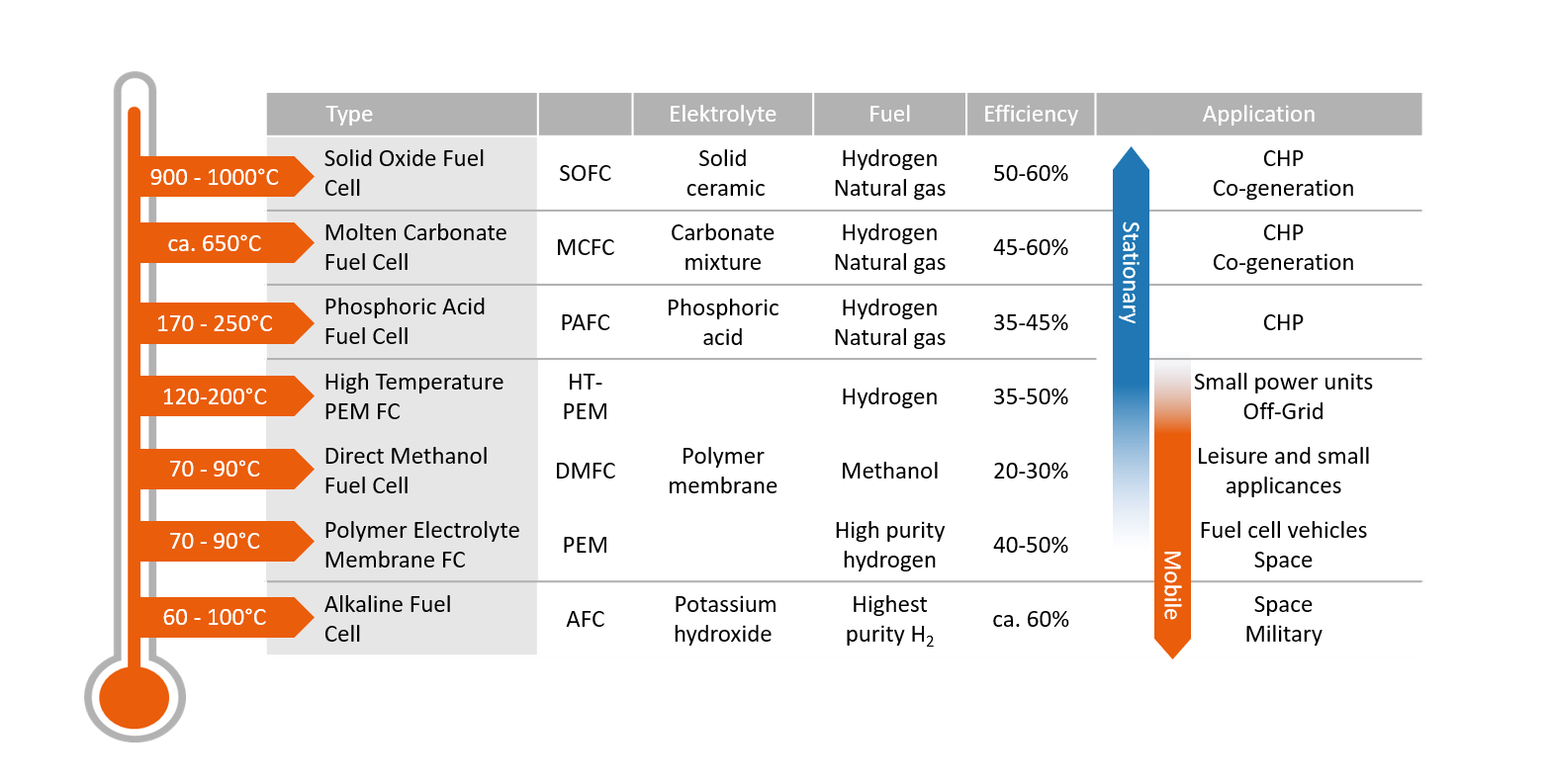

Comparison of different types of fuel cells
Alkaline Fuel Cell
AFC
In the alkaline fuel cell, watery potassium hydroxide solution serves as the electrolyte. Due to the low corrosiveness of the electrolyte, low-cost materials can be used for the system design. Since potassium hydroxide reacts with carbon dioxide to form an insoluble carbonate and thus impairs the chemical reaction, the hydrogen used must be highly pure (99.999 %). The high technical cost of gas treatment currently limits the field of application to military and space.
Polymer Electrolyte Membrane Fuel Cell
PEMFC
A solid polymer membrane serves as the electrolyte in the PEM fuel cell. This guarantees the particularly flexible behavior during load changes. PEM fuel cells start up very well and quickly – this makes them suitable for vehicle powertrains as well. In addition, waste heat can be used in decentralized power systems. At low temperatures, however, PEM fuel cells require a high proportion of precious metal catalysts (usually platinum) and complex water management to prevent the membrane from drying out. They are susceptible to carbon monoxide contamination – thus requiring high-purity hydrogen (min. 99.97%).
Direct Methanol Fuel Cell (based on PEM)
DMFC
Direct methanol fuel cells also incorporate a polymer membrane as the electrolyte. Liquid methanol is used as the fuel. The liquid energy carrier is easier to handle, transport, and store than gaseous hydrogen. However, DMFCs are severely limited in terms of power and scalability. Their field of application is therefore limited to small appliances and camping applications. Currently, a high catalyst content (platin) is required. The reduction of methanol to carbon monoxide further limits the catalyst’s functionality.
High Temperature Polymer Electrolyte Membrane
HT-PEMFC
The HT-PEM is an advancement of the PEM fuel cell, which is operated at a temperature of up to 200°C. In contrast to the standard, low temperature PEM (LT-PEM), the membranes do not have to be kept moist. In addition, the higher temperature reduces the requirements regarding purity of the fuel gas used. It is thus possible to use hydrogen, which is reformed on site from a liquid hydrogen carrier such as methanol, without any great technical effort. In contrast to the low-temperature variant, HT-PEMs have a reduced dynamic load behavior and need longer to start-up.
Phosphoric Acid Fuel Cell
PAFC
Phosphoric acid fuel cells operate with phosphoric acid as the electrolyte. The acid is bound in a fiber structure made of plastic. In addition to hydrogen, carbon-containing gases such as natural gas are also used. Due to a long heat-up time of up to 3 hours, phosphoric acid fuel cells are particularly suitable for decentralized energy supply to serve a base load. To prevent crystallization of the electrolyte, PAFCs must be kept permanently warm at around 50 °C – even in standby.
Molten Carbonate Fuel Cell
MCFC
Molten carbonate fuel cells use a mixture of potassium and lithium carbonate as electrolyte. In addition to hydrogen, the use of natural gas and biogas is possible. Due to the high temperature, expensive precious metal catalysts are not necessary, but the materials used also have to be more resistant to heat. In addition, the corrosive electrolyte attacks many materials. Since a start-up process takes several hours and constant heating and cooling leads to wear and tear of the system, MCFCs are primarily used for base-load energy supply. High exhaust gas temperatures make the system interesting for co-generation of power and heat.
Solid Oxide Fuel Cell
SOFC
In solid oxide fuel cells, the electrolyte consists of a solid ceramic material. As with MCFCs, other carbon-containing gases can be used besides hydrogen. However, high operating temperatures also place high demands on the materials used in SOFCs.
By the way: the process of a fuel cell can also be reversed. This allows water to be broken down into hydrogen and oxygen with the help of electric current. A process referred to as electrolysis.
Fuel cell advantages and disadvantages
As energy converters, fuel cells are efficient and environmentally friendly. Compared to combustion engines, however, they are – as of today – also significantly more expensive. In combination with batteries, high power and high energy density can be optimally combined.
Fuel cell vs diesel generator
In a diesel generator, heat is generated by burning a fuel. The heat is converted into motion (mechanical energy) and ultimately into electrical energy. In contrast, fuel cells convert the chemical energy from the fuel directly into electrical energy. As a result, they work much more efficiently:
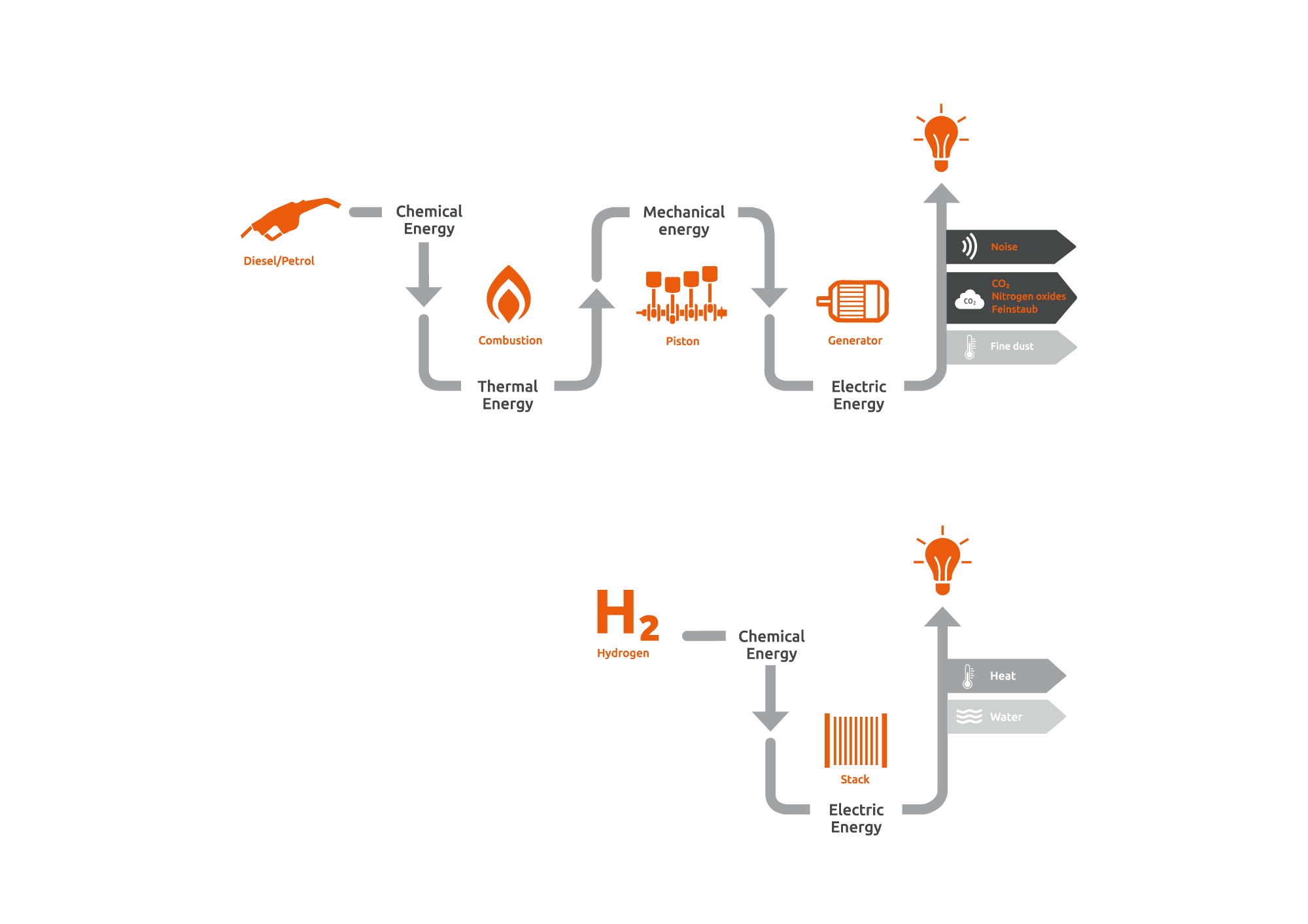
Fuel cell and diesel generator comparison
In contrast to conventional fuel combustion, neither particulate matter nor nitrogen oxides are emitted. Since fuel cells have no moving parts, they operate silently and require little maintenance.
Advantages
- Long operating times without maintenance
- No abrasion in stand-by
- No emissions
- High electrical efficiency
- Silent
- Modular scalable
Disadvantages
- (Partially) low field experience
- High investment cost for high power
- Fewer suppliers in the market
- Challenges regarding storage and supply of hydrogen
Advantages and disadvantages of fuel cells compared to diesel generators
However, widespread use of fuel cells is currently still limited by high investment costs. In addition, fuel cells operate particularly efficiently with hydrogen. However, green hydrogen is hardly available at present and has a relatively low energy density by volume compared to conventional fuels. This makes fuel supply difficult, especially in remote areas.
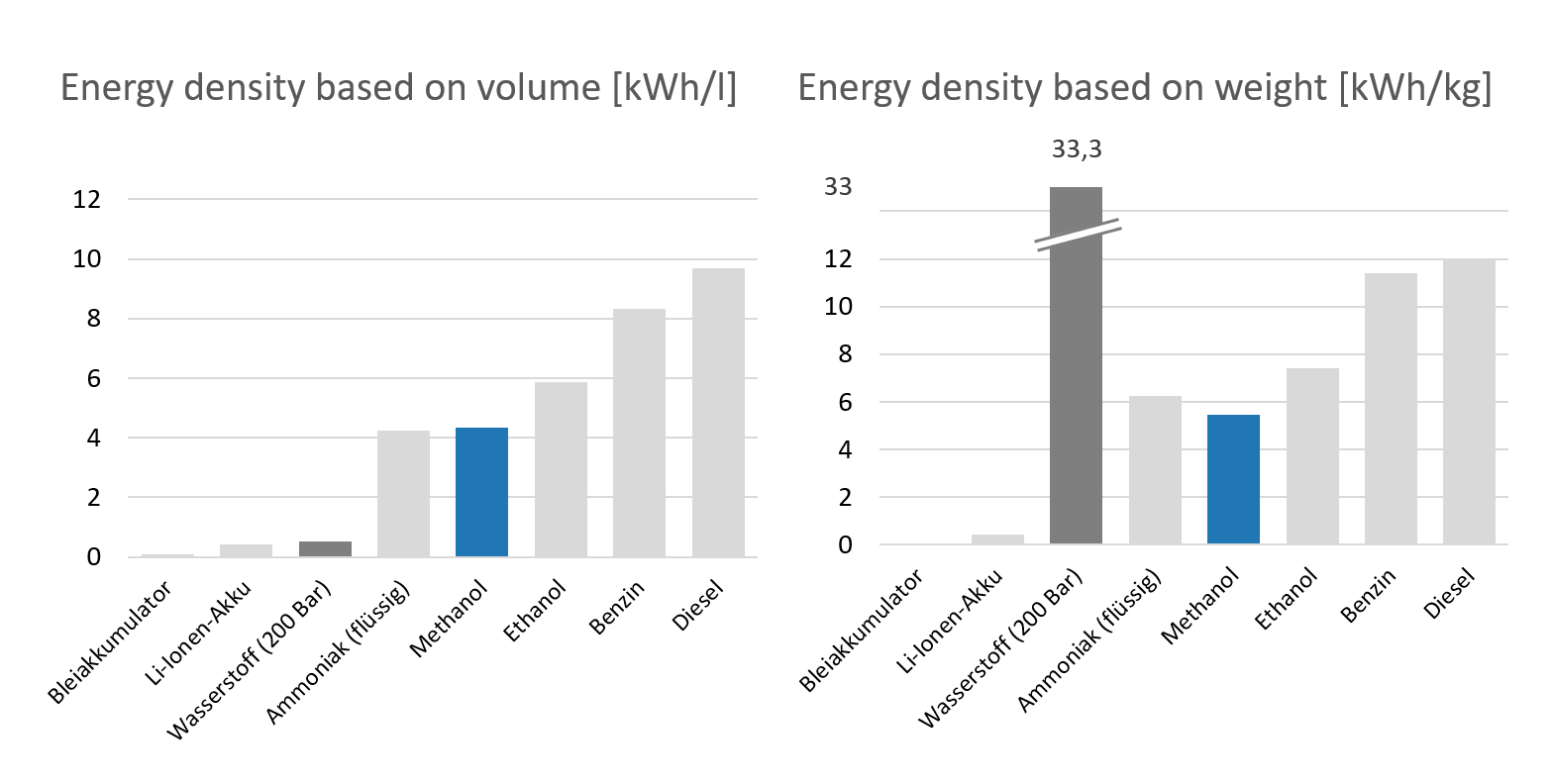

Energy density of different types of fuels
Liquid hydrogen carriers such as methanol represent a possible solution. Although their energy density is lower than that of diesel or gasoline, the high efficiency of the fuel cell often still results in reduced fuel consumption. Unlike diesel and gasoline, methanol can be obtained relatively easy from renewable sources or biomass.
Fuel cell vs battery
In fuel cells, energy is not stored inside the cell but supplied externally via the fuel. While batteries are heavy and bulky at high capacity, the capacity of a fuel cell can be easily adjusted via the external tank.
The relationship between capacity and weight can be observed in battery-powered electric cars: To achieve a long range, as many battery packs as possible need to be installed. However, additional packs result are extra weight for the vehicle, which in turn limits the range.
Advantages
- Low weight and compact design
- Fast refueling
Disadvantages
- High investment cost for high power
- Challenges regarding storage and supply of hydrogen
Advantages and disadvantages of fuel cells compared to batteries
On the other hand, fuel cells are presently still associated with high costs – especially at high power outputs. The supply of hydrogen is often difficult. A solution is promised by liquid renewable fuels such as methanol, in which hydrogen is chemically bound.
Instead of thinking of fuel cells and batteries as distinct entities, the two technologies are gradually being combined. In so-called hybrid systems, fuel cells and batteries work in synergy. The high power density of batteries is combined with the high energy density of the fuel used.
Applications of fuel cells
The first applications of fuel cells date back to space travel and the military. At the time, cost was hardly an issue, but the technological advantages of high energy density and quiet operation were paramount for operational success.
Applications are generally divided up into portable, mobile and stationary applications. However, due to advancements in battery technology, fuel cells are hardly of any importance for the portable supply of small electrical consumers (laptops, cameras, etc.) today.
Off-grid, backup power, residential energy: Fuel cells in stationary applications
Stationary applications include systems for continuous power supply, emergency power applications, but also the combined generation of heat and electricity. Due to their low-maintenance and emission-free operation, fuel cells already represent an economical alternative to conventional generators in some areas.
In residential energy supply, fuel cell units generate heat and electricity at the same time. PEMFCs, as well as SOFCs, are mostly used in small scale systems- with hydrogen or natural gas being used as fuel.
In contrast to private homes, industrial plants, hotels and hospitals have significantly higher energy demands. This can be met by fuel cell-powered combined heat and power (CHP) plants, which have the potential to gradually replace engine-powered plants due to their better efficiency and elimination of pollutants. MCFCs and SOFCs are predominantly used, operating at high temperatures with natural gas.


SIQENS stationary fuel cell system in an office container (Foto: Stefan Stark)
In backup power systems, fuel cells combine the advantages of battery and diesel solutions. Depending on the fuel, power generation is emission-free. Long outages can be bridged by sizing the fuel tank as needed. Thanks to low maintenance costs, the initially higher investment usually amortizes within a few years compared to diesel gensets.
Fuel cell systems are often used commercially for off-grid power supply. In combination with photovoltaics, fuel cells are suitable for bridging supply gaps in solar coverage.
In commerical environments, PEMFCs and DMFCs are used in security and surveillance technology, measuring stations, and telecommunications. Since systems often remain transportable, the distinction between stationary and mobile systems becomes increasingly blurred.
Cars and vehicles: fuel cells in mobility applications
Mobility applications of fuel cells include vehicle drivetrains, but also auxilliarypower supply. Often, PEMFCs are employed as they can be switched on and off frequently. Since the powertrain is always electric, fuel cell cars are by definition electric vehicles.
Based on the relationship between capacity and weight described above, long ranges for battery-powered electric cars are often achieved at cost of heavy and bulky battery technology. The additional weight brought in via batteries in turn is not optimal when aiming to achieve long range.


SIQENS fuel cell as range extender demonstrator (Foto: Dino Eisele)
To address this challenge, fuel cells can be used as range extenders in battery-powered vehicles. Instead of providing the energy required by the drivetrain solely via batteries, the fuel cell converts the chemical energy stored as fuel inside a tank into electricity to cover long driving distances.
As an auxiliary power unit (APU), fuel cells offer the possibility of providing electricity on board independently of the battery. Fuel cells are therefore used in commercial vehicles, for example when electrifying cooling vehicles.
In addition, waste heat can be used to heat the vehicle without burdening the battery. Once again, the advantage lies within the high energy density and the quick refueling capability.
Fuel cells with methanol
Methanol is an interesting fuel, especially in terms of energy density and simplicity of logistics. With regard to its use in fuel cells, a distinction can be made between two modes of operation: Fuel cells that use methanol directly and those that use methanol indirectly.
Direct methanol fuel cells (DMFC) are fed with a methanol-water mixture at the anode side. With 20-30% their efficiency is relatively low compared to other fuel cells (link to types of fuel cells). Their field of application is usually limited to the supply of small appliances in the leisure sector.
Fuel cells that use methanol indirectly require a reformer to dissolve the hydrogen that is chemically-bound inside methanol. This happens at a temperature of 200 – 220°C. Thus, the fuel cell stack is fed with gaseous hydrogen rather than liquid methanol. In this respect, methanol only serves as a liquid hydrogen carrier.
>>> More on the topic of methanol fuel cells in our blog entry
More on SIQENS Fuel cells.