As a clean alternative to fossil-fueled combustion engines, fuel cells are gaining growing attention. Yet much of the focus remains on operation with gaseous hydrogen. But there are also alternative concepts: one of them is the methanol fuel cell.
1. Design and principle of a methanol fuel cell
2. Direct methanol fuel cell (DMFC)
3. Indirect methanol fuel cell (RMFC)
4. Advantages of methanol for fuel cells
Design and principle of a methanol fuel cell
Similar to any other type of fuel cell, methanol fuel cells consist of two electrodes (anode and cathode), which are separated from each other through an electrolyte. The electrodes are conductive for electrons, while the electrolyte is only permeable for positively charged hydrogen atoms.
Typically, methanol fuel cell systems build on a polymer electrolyte membrane (PEM). In terms of design, a distinction can be made between two modes of operation: Fuel cells that use methanol directly and those that use methanol indirectly.
More on different fuel cell types
Direct methanol fuel cell (DMFC)
Direct methanol fuel cells (DMFC) are the most common form of methanol fuel cell. Compared to other methanol fuel cells, they are characterized by a simple system design and fast start-up times. They are usually low-power systems with an output of < 200 Watts.
Electrical efficiency: 20 – 30%
Operating temperature: 70 – 90°C
Reaction: 2 CH3OH + 3 O2 -> 4 H2O + 2 CO2
Design and principle
DMFCs supply a methanol-water mixture at the anode side. The mixture is split into hydrogen and carbon dioxide. A catalyst (platinum) divides the hydrogen molecules into positively charged hydrogen atoms (protons).
The hydrogen protons pass through the electrolyte (a proton exchange membrane) to the cathode and react with oxygen to form water. The process takes place at a comparatively low cell temperature of 70 to 90°C, which enables fast start-up times.

Principle and basic design of a direct methanol fuel cell (DMFC) – Schematic illustration
Applications
DMFCs are used both in commercial and private settings. The fields of application include remote monitoring stations, video surveillance systems or smaller electrical systems in traffic control – mostly with a demanded power of less than 150 Watts. In the leisure sector, DMFCs are used for on-board power supply in caravans and boats.
Advantages and disadvantages
Besides the general advantages of fuel cells over conventional combustion engines, direct methanol fuel cells are characterized by a simple system design and fast start-up times. However, DMFCs are relatively sensitive to impurities in the fuel and have a relatively low efficiency.
Advantages
- Fast start-up time
- Simple and compact system design
- Widely used and well-established for low power applications
Disadvantages
- Require high purity methanol
- Comparatively low efficiency (20 – 30%)
- Storage temperature at sub-zero temperatures problematic, as formation of water crystals can damage the membrane
- Methanol “cross-over” on membrane can affect lifetime and system efficiency
- High platinum content required as catalyst
Advantages and disadvantages of direct methanol fuel cells (DMFCs) compared to other methanol fuel cells
Indirect methanol fuel cells (RMFC)
The indirect methanol fuel cell (also reformed methanol fuel cell or RMFC) uses hydrogen as fuel. The hydrogen is extracted from methanol in a pre-process. This system design allows for a higher power output and improved electrical efficiency compared to DMFCs.
Electrical efficiency: 35 – 50%
Operating temperature: depending on membrane 70 – 90°C (LT-PEM) or 160 – 200 °C (HT-PEM)
Reaction: CH3OH + H2O -> 3 H2 + CO2 (Reformer) 2 H2 + O2 -> 2 H2O (Fuel cell)
Design and principle
RMFCs can differ with regard to their membrane. Some systems use conventional low-temperature polymer electrolyte membranes (LT-PEM), while others use high-temperature polymer electrolyte membranes (HT-PEM). More on the different types here. However, the basic working principle is similar:
In the first step, methanol is converted into a hydrogen-containing gas (reformate gas). This so-called steam reformation process takes place at temperatures between 200 – 220°C. More about the process can be found below in the text. Methanol serves as a liquid hydrogen carrier.
In contrast to a DMFC, no watery methanol is fed to the anode, but gaseous hydrogen. However, since LT-PEMs – a fuel cell type commonly used in the automotive industry – require high-purity hydrogen, the reformate gas must be purified before being used for power generation. This may be accomplished by means of a palladium membrane.
In contrast to LT-PEMs, HT-PEMs are tolerant to impurities in the reformate gas due to their elevated operating temperature. The step of conditioning is therefore not required for this system design.
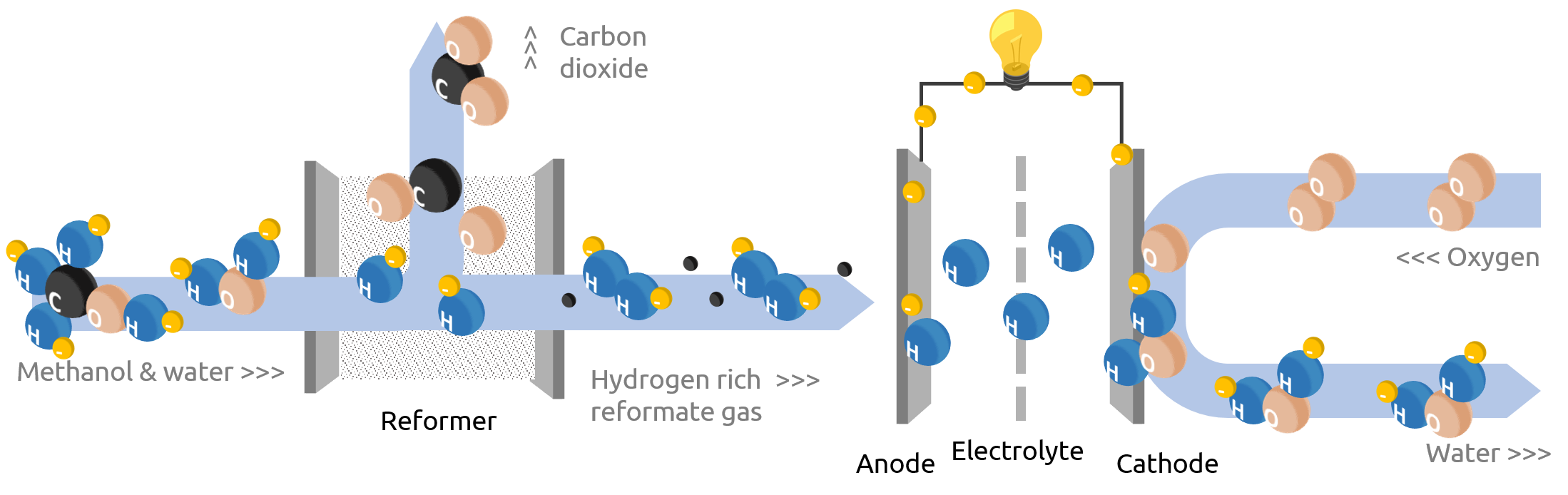
Principle and basic design of an indirect methanol fuel cell (RMFC) with reformer and HT-PEM (LT-PEM requires an additional step of purification between fuel cell and reformer) – Schematic illustration
Applications
RMFCs are predominantly used for stationary power generation, as well as in the automotive sector. Fields of application include remote telecommunication sites or backup power supply for critical infrastructure. Systems and modules vary from 150 watts to several hundred kilowatts, depending on the field of application.
Advantages and disadvantages
Compared to a DMFC, the system design of the RMFC is more complex. However, the requirements regarding the methanol purity are lower. In addition, a higher efficiency can be achieved.
Advantages
- High efficiency (35- 50%)
- Cold storage since no liquid is in direct contact with the membrane
- Lower platinum content
- Use of industrial methanol (for example according to IMPCA)
Disadvantages
- Slower startup time due to warm-up phase
- More complex system design
Advantages and disadvantages of indirect methanol fuel cells (RMFCs) compared to DMFCs
![]()
All methanol fuel cells require a methanol-water mixture. However, since water (vapor) is formed on the cathode side of the fuel cell during operation, this can be condensed out and mixed with pure methanol. For this reason, some manufacturers allow the use of pure methanol as a fuel.
Advantages of methanol for fuel cells
Containing four hydrogen, one carbon, and one oxygen atom, methanol (H3COH) is the simplest organic alcohol. High energy density and easy production from renewable energy sources or biomass make methanol an interesting fuel for future energy systems.
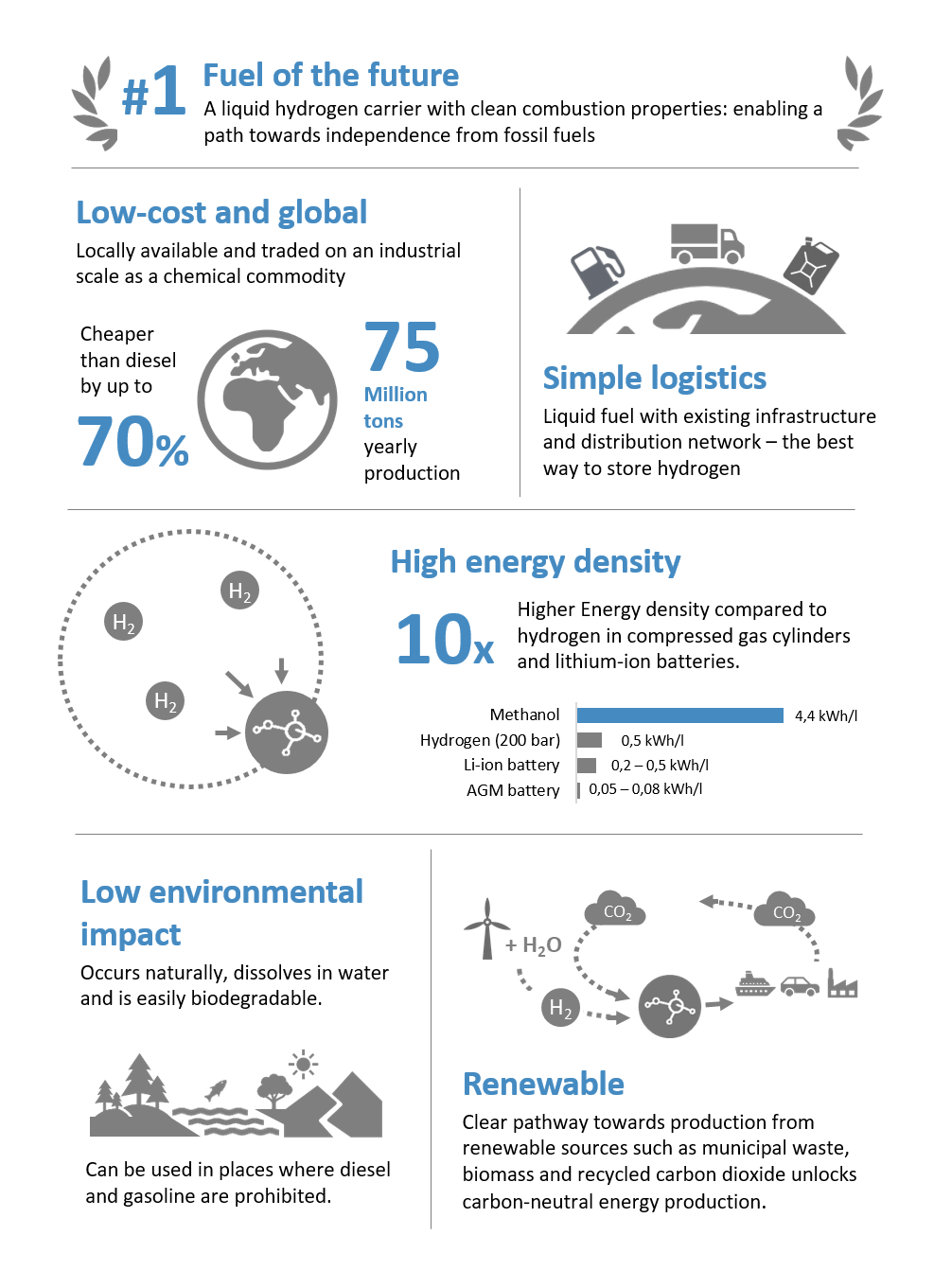
Advantages of methanol for fuel cells
Transport and storage
Methanol is liquid at room temperature and can be transported and stored in tanks, canisters or barrels. In addition, it has a low freezing point of – 97°C, making methanol fuel cells suitable for use in cold ambient temperatures.
Low cost and globally available
Methanol is an important feedstock for the chemical industry and is used, for instance, as a solvent. Due to its versatility, methanol is traded globally. In Europe, the net price for methanol – supplied in an IBC – is around €0.50 per liter.
High energy density
Methanol has a high proportion of chemically bonded hydrogen and is characterized by its high energy density. To put this into perspective: 10 liters of methanol contain approximately 1 kilogram of hydrogen. A 25 liter canister consequently contains the same amount as 2 pressurized gas cylinders with gaseous hydrogen at 200 bar.
Production of hydrogen from methanol
Today, hydrogen is mainly produced from hydrocarbons. This is a group of compounds consisting only of carbon (C) and hydrogen (H). These include fossil fuels such as natural gas, coal and petroleum, but also alcohols such as methanol and ethanol (C2H4O).
The most common process is that of steam reforming. In this process, the chemically bound hydrogen is extracted from the source materials at high temperature. The result is a reformate gas rich in hydrogen.
In contrast to other hydrocarbons, methanol can be converted into a hydrogen-containing reformate gas at a relatively low temperature of 200 – 220 °C. For power generation, the reformate gas can be used directly in HT-PEM fuel cells.
Purification of hydrogen allows for usage of hydrogen in industrial processes or in other types of fuel cells. Today, this can be accomplished by Pressure Swing Adsorption (PSA), Palladium membranes, or Electrochemical Hydrogen Separation (EHS).
More on SIQENS fuel cells.